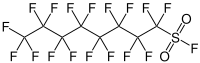
Perfluorooctanesulfonyl fluoride
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-heptadecafluorooctane-1-sulfonyl
fluoride | |
| Identifiers | |
| 307-35-7 | |
| 3D model (Jmol) | Interactive image |
| Abbreviations | POSF, PFOSF |
| ChemSpider | 9019 |
| ECHA InfoCard | 100.005.638 |
| EC Number | 206-200-6 |
| PubChem | 9388 |
| |
| |
| Properties | |
| C8F18O2S | |
| Molar mass | 502.12 g/mol |
| Boiling point | 154 °C (309 °F; 427 K)[1] |
| Related compounds | |
| Related compounds |
Perfluorooctanesulfonic acid (PFOS), Perfluorooctanesulfonamide (PFOSA), Perfluorooctanoic acid (PFOA), Perfluorobutanesulfonic acid (PFBS) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Perfluorooctanesulfonyl fluoride (POSF) is a synthetic perfluorinated compound with a sulfonyl fluoride functional group. It is used to make perfluorooctanesulfonic acid (PFOS) and PFOS-based compounds. These compounds have a variety of industrial and consumer uses, but POSF-derived substances ultimately degrade to form PFOS.
Because of environmental concerns over PFOS, 3M ceased POSF use in 2002 and global production plummeted. However, Chinese production grew after 3M's phaseout. As of May 2009, POSF and PFOS are listed as persistent organic pollutants (POPs) included in Annex B of the Stockholm Convention.
Production
In 1949, 3M began producing POSF by electrochemical fluorination (ECF).[2] From 1966 to the 1990s, 3M production increased to meet demand for POSF-based compounds.[2] In 1999, 3M reported POSF was its most highly produced fluorochemical.[3] Before 2000, 3M was the largest global producer of POSF (mainly at their Decatur, AL and Antwerp facilities) and global production peaked at ~4500 tonnes per year.[2]
In 1999, the U.S. Environmental Protection Agency began investigating perfluorinated compounds after receiving data on the global distribution and toxicity of PFOS, the key ingredient in Scotchgard.[4] For these reasons, and USEPA pressure,[5] the primary American producer of PFOS, 3M, announced, in May 2000, the phaseout of the production of PFOS, PFOA, and PFOS-related products.[6] 3M stated that they would have made the same decision regardless of USEPA pressure.[7]
Immediately after the 2000–2002 3M phaseout, production plummeted, but dominant and growing production shifted to China.[8] In 2004 Chinese production of PFOS-based compounds was estimated to be <50 tonnes.[8] In 2005 global production was estimated to be between 73–162 tonnes,[9] and by 2006 Chinese production was estimated at >200 tonnes.[8] Total historical global production was estimated at ~120,000 tonnes in 2009.[2]
"[M]ost, if not all" industrially synthesized perfluorooctanesulfonyl derivatives, such as PFOS, have POSF as their precursor.[10]
Synthesis
POSF is synthesized by electrochemical fluorination of octanesulfonyl fluoride in anhydrous hydrogen fluoride by the equation:[10]
- C8H17SO2F + 17 F− → C8F17SO2F + 17 H+ + 34 e−.
This reaction results in a 25% yield for POSF, less than that for shorter perfluorosulfonyl fluorides.[10] The POSF obtained is impure as it is a mixture of linear and branched isomers, with ∼70% linear.[10] POSF can also be obtained by ECF of the sulfonyl halide octanesulfonyl chloride.[10]
Reactivity
POSF and POSF-based polymers degrade to form PFOS[11] which is not known to degrade by any environmental processes.[5] POSF hydrolysis in water, however, occurs slowly.[10]
POSF reacts with bases such as potassium hydroxide to form PFOS salts:[10]
- C8F17SO2F + KOH → C8F17O2SO3−K+.
Upon treatment with sulfuric acid the sulfonic acid PFOS tetrahydrate is obtained.[10]
POSF also reacts with ammonia to form perfluorooctanesulfonamide:[10]
- C8F17SO2F + NH3 → C8F17O2SNH2.
Sulfonamides and sulfonamidoethanols synthesized from POSF can in turn react to form a variety of different functional groups for different applications and products.[12]
Uses
Because of multiple carbon–fluorine bonds, POSF-derivatives have chemical properties that are hydrophobic ("water-afraid"), lipophobic ("fat-afraid"), and surface tension lowering (as fluorosurfactants).[3] The main uses of chemical substances derived from POSF have been:[2]
- as stain and soil repellents for carpet;
- as water repellents for clothing;
- as fat and oil repellents in food packaging;
- as specialty applications such as semiconductor manufacturing and hydraulic fluids for airplanes; and
- in aqueous film forming foam (fire fighting foam) as fluorosurfactants.
The Stockholm Convention lists a variety of acceptable purposes and specific exemptions for POSF and PFOS (and it salts) including
- photo-imaging;
- photo-resist and anti-reflective coatings for semiconductors;
- etching agent for compound semiconductors and ceramic filters;
- aviation hydraulic fluids;
- metal plating (hard metal plating) only in closed-loop systems;
- certain medical devices (such as ethylene tetrafluoroethylene copolymer (ETFE) layers and radio-opaque ETFE production, in-vitro diagnostic medical devices, and CCD colour filters);
- fire fighting foam;
- insect baits for control of leaf-cutting ants from Atta spp. and Acromyrmex spp.;
- photo masks in the semiconductor and liquid crystal display (LCD) industries;
- metal plating (hard metal plating);
- metal plating (decorative plating);
- electric and electronic parts for some colour printers and colour copy machines;
- insecticides for control of red imported fire ants and termites;
- chemically driven oil production;
- carpets;
- leather and apparel;
- textiles and upholstery;
- paper and packaging;
- coatings and coating additives; and
- rubber and plastics.[13]
International status
At the Fourth Conference of Parties, decision SC-4/17 put POSF, along with PFOS, in the Stockholm Convention on Persistent Organic Pollutants (Annex B) in May 2009.[14][15] As such, POSF is not "banned" but has approved uses and exemptions—along with a program (SC-4/19) in Annex B that encourages reduced production.[16][17]
Environmental concern
The POSF degradation product, PFOS, is the dominant perfluorinated compound detected in biomonitoring studies,[18] where concentrations that have been detected are considered sufficient to "alter health parameters".[19][20]
See also
References
- ↑ "Perfluoro-1-octanesulfonyl fluoride". National Institute of Standards and Technology. Retrieved 10 July 2009.
- 1 2 3 4 5 Paul AG, Jones KC, Sweetman AJ (2009). "A first global production, emission, and environmental inventory for perfluorooctane sulfonate". Environ. Sci. Technol. 43 (2): 386–92. doi:10.1021/es802216n. PMID 19238969.
- 1 2 (1999) 3M. 1999. The science of organic fluorochemistry. 3M Company, February 5, 1999. (PDF readily accessible via a google search for the article title.)
- ↑ Aziz Ullah. "The Fluorochemical Dilemma: What the PFOS/PFOA fuss is all about" Cleaning & Restoration. www.ascr.org, (October, 2006). Accessed October 25, 2008.
- 1 2 Lee, Jennifer 8. (15 April 2003). "E.P.A. Orders Companies to Examine Effects of Chemicals". The New York Times. Retrieved 15 May 2009.
- ↑ 3M: "PFOS-PFOA Information: What is 3M Doing?" Accessed October 25, 2008.
- ↑ Weber, Joseph (5 June 2000). "3M's Big Cleanup – Why it decided to pull the plug on its best-selling stain repellant". Business Week. New York: McGraw-Hill (3684): 96.
- 1 2 3 Wang T, Wang Y, Liao C, Cai Y, Jiang G (2009). "Perspectives on the Inclusion of Perfluorooctane Sulfonate into the Stockholm Convention on Persistent Organic Pollutants". Environ. Sci. Technol. 43 (14): 5171–5. doi:10.1021/es900464a. PMID 19708337.
- ↑ (December 2006). "Results of the 2006 OECD Survey on Production and Use Of PFOS, PFAS, PFOA, PFCA, Their Related Substances and Products/Mixtures Containing These Substances" (PDF). Organisation for Economic Co-operation and Development. p. 10. Retrieved 30 August 2009.
- 1 2 3 4 5 6 7 8 9 Lehmler, HJ (2005). "Synthesis of Environmentally Relevant Fluorinated Surfactants – A Review". Chemosphere. 58 (11): 1471–96. doi:10.1016/j.chemosphere.2004.11.078. PMID 15694468.
- ↑ Giesy JP, Kannan K (2002). "Perfluorochemical Surfactants in the Environment". Environ. Sci. Technol. 36 (7): 146A–152A. doi:10.1021/es022253t. PMID 11999053.
- ↑ "Hazard Assessment of Perfluorooctane sulfonate (PFOS) and its Salts" (PDF). Organisation for Economic Co-operation and Development. 21 November 2002. p. 14.
- ↑ "Report of the Conference of the Parties of the Stockholm Convention on Persistent Organic Pollutants on the work of its fourth meeting" (PDF). p. 67.
- ↑ "Governments unite to step-up reduction on global DDT reliance and add nine new chemicals under international treaty". Geneva: Stockholm Convention Secretariat. 8 May 2008.
- ↑ "Report of the Conference of the Parties of the Stockholm Convention on Persistent Organic Pollutants on the work of its fourth meeting" (PDF). Geneva. 8 May 2009. p. 66.
- ↑ "Report of the Conference of the Parties of the Stockholm Convention on Persistent Organic Pollutants on the work of its fourth meeting" (PDF). pp. 67–69.
- ↑ "U.N. treaty expanded by 9 more chemicals: Participating nations get one year to decide on next steps". MSNBC. Associated Press. 11 May 2009. Retrieved 5 September 2009.
- ↑ Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC (June 2006). "Biological monitoring of polyfluoroalkyl substances: A review". Environ. Sci. Technol. 40 (11): 3463–73. doi:10.1021/es052580b. PMID 16786681. Supporting Information (PDF).
- ↑ Peden-Adams, M. M.; Keil, D. E.; Romano, T.; Mollenhauer, M. A. M.; Fort, D. J.; Guiney, P. D.; Houde, M.; Kannan, K.; Muir, D. C.; Rice, C. D.; Stuckey, J.; Segars, A. L.; Scott, T.; Talent, L.; Bossart, G. D.; Fair, P. A.; Keller, J. M. (2009). "Health effects of perfluorinated compounds—What are the wildlife telling us?". Reproductive Toxicology. 27 (3–4): 414. doi:10.1016/j.reprotox.2008.11.016.
- ↑ Peden-Adams et al. (June 2008). In PFAA Days II (PDF). p. 28.
External links
- PFOS Information submitted to the Stockholm Convention.
- Perfluorooctane Sulfonyl Fluoride as an Initiator in Hot-Filament Chemical Vapor Deposition of Fluorocarbon Thin Films (PDF).