Kynapcin
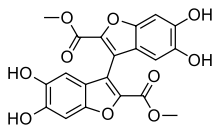
Skeletal formula of kynapcin-24
Kynapcin is a general name for a number of dibenzofuranyl derivatives of the molecule polyozellin, present in the fungus Polyozellus multiplex. Like polyozellin, it inhibits prolyl endopeptidase, an enzyme that has a role in processing proteins (specifically, amyloid precursor protein) in Alzheimer's disease. Chemicals that inhibit prolyl endopeptidase have attracted research interest due to their potential therapeutic effects.[1] Several kynapcins have been found in P. multiplex, each with different chemical properties, including kynapcin-12,[2] kynapcin-13 and -28,[3] and -24.[4] A total synthesis of kynapcin-24 was achieved in 2009.[5]
References
- ↑ Hwang JS, Song KS, Kim WG, Lee TH, Koshino H, Yoo ID (1997). "Polyozellin, a new inhibitor of prolyl endopeptidase from Polyozellus multiplex". The Journal of Antibiotics. 50 (9): 773–777. doi:10.7164/antibiotics.50.773. PMID 9360624.
- ↑ Lee HJ, Rhee IK, Lee KB, Yoo ID, Song KS (2000). "Kynapcin-12, a new p-terphenyl derivative from Polyozellus multiplex, inhibits prolyl endopeptidase". The Journal of Antibiotics. 53 (7): 714–719. doi:10.7164/antibiotics.53.714. PMID 10994814.
- ↑ Kim SI, Park IH, Song KS (2002). "kynapcin-13 and -28, new benzofuran prolyl endopeptidase inhibitors from Polyozellus multiplex". The Journal of Antibiotics. 55 (7): 623–628. doi:10.7164/antibiotics.55.623. PMID 12243451.
- ↑ Song KS, Raskin I (2002). "A prolyl endopeptidase-inhibiting benzofuran dimer from Polyozellus multiflex". Journal of Natural Products. 65 (1): 76–78. doi:10.1021/np010194b. PMID 11809072.
- ↑ Yang LY, Chang CF, Huang YC, Lee YJ, Hu CC, Tseng TH (2009). "The first total synthesis of kynapcin-24 by palladium catalysis". Synthesis-Stuttgart. 7: 1175–1179. doi:10.1055/s-0028-1087998.
This article is issued from Wikipedia - version of the 11/28/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.